How To Make Paint With Chemicals
Paint is used to decorate, protect and prolong the life of natural and synthetic materials, and acts as a bulwark confronting environmental conditions.
Paints may be broadly classified into Decorative paints, applied on site to decorate and protect buildings and other objects, and Industrial coatings which are applied in factories to finish manufactured appurtenances such equally cars.
The constituents of paint
Paints incorporate:
- pigment(s) - prime pigments to impart colour and opacity
- binder (resin) - a polymer, ofttimes referred to as resin, forming a matrix to hold the pigment in place
- extender - larger pigment particles added to ameliorate adhesion, strengthen the motion picture and save binder
- solvent (sometimes called a thinner) - either an organic solvent or water is used to reduce the viscosity of the paint for better application. H2o-borne paints are replacing some paints that use volatile organic compounds such equally the hydrocarbons which are harmful to the atmosphere.
- additives - used to alter the properties of the liquid paint or dry picture
The folder (resin) and solvent together are sometimes known equally the vehicle. The folder may exist dissolved as a solution or carried every bit a dispersion of microscopically small-scale particles in a liquid.
Depending on the blazon of paint and intended use, additives may include:
- dispersants - to dissever and stabilise pigment particles
- silicones - to improve conditions resistance
- thixotropic agents - to give paints a jelly-like consistency that breaks downward to a liquid when stirred or when a brush is dipped into it
- driers - to accelerate drying fourth dimension
- anti-settling agents - to prevent pigment settling
- bactericides - to preserve water based paints in the can
- fungicides and algaecides - to protect exterior paint films against disfigurement from moulds, algae and lichen
Paints are formulated according to their proposed use - primer, undercoat, special finishes (matt, gloss, heat resistance, anti-corrosion, abrasion resistance). The pigment powder is broken downwardly into individual particles which are coated by and dispersed in the binder (resin) - known every bit 'wetting out'. Solvent is and then added to give the required consistency. Each batch of ingredients is thoroughly mixed in large, stirred containers with the required additives (Figure 1). Amounts ranging upward to 40 000 dm3 of paint may be fabricated in a unmarried batch.
| Figure ane Contents of a white gloss (alkyd) pigment and a white matt emulsion (acrylic) paint. | .jpg) |
This unit of measurement discusses the well-nigh commonly used binders followed past the pigments.
Binders in paints
The three nigh important binders (resins) used in modern paints are:
- acrylic polymers (resins)
- alkyd polymers (resins)
- epoxy polymers (resins)
Acrylic polymers (resins)
The binder in many emulsion paints is based on homopolymers or co-polymers of ethenyl ethanoate (vinyl acetate) and a propenoate (acrylic) ester.
Ethenyl ethanoate is manufactured by passing a mixture of ethanoic acid vapour, ethene and oxygen over heated palladium(ll) and copper(ll) chlorides:
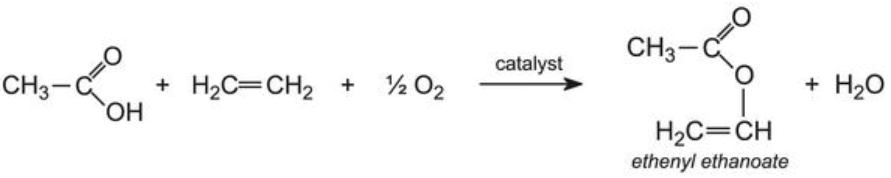
Ethenyl ethanoate and an acrylic ester (for example, methyl 2-methylpropenoate) are and so co-polymerized to course a random assortment, in which these groups link into a linear chain:
.jpg)
Other acrylic esters used every bit co-monomers with ethenyl ethanoate are ethyl propenoate, butyl propenoates, or a co-polymer of butyl propenoate and methyl ii-methylpropenoate.
The polymers used in these paints are carried in water (h2o-borne emulsion paints) which as described above are much meliorate for the environment than paints in which the binders are in organic solvents.
| Figure ii H2o-borne emulsion paints are used as decorative paints, particularly for the inside and outside of buildings (including masonry paints and exterior primers). By kind permission of AkzoNobel. |  |
Emulsion paints are so-called as they are made past a process known as emulsion polymerization, in which the liquid monomers to be polymerized are first dispersed in water, as an emulsion. The polymers produced by this process typically have relative molecular masses of 500 000 - 1 000 000. Every bit such they are useful merely equally dispersions since they would be extremely viscous if they were carried in solution and this would brand them unusable.
.jpg)
Figure 3 Graph showing relationship between relative molecular
mass and viscosity for solution and dispersion polymers.
Acrylic resins may also exist used in industrial paints, either as water-borne emulsion paints or as solvent-borne paints. Solvent-borne industrial paints tin can take a tough protective terminate and are widely used in industry equally topcoats, for example on automobile bodies. The paint oftentimes comes as ii components which are mixed together just before use: the chief paint portion typically consists of an acrylic resin produced past the polymerization of a propenoate ester formed from a polyhydric alcohol (diols and triols). The resulting polyester has numerous hydroxyl groups (-OH) pendant from the polymer backbone. The hydroxyl groups react with the other chemical compound often consisting of a polymeric isocyanate such as a trimer of 1,6-diisocyanatohexane (hexamethylene diisocyanate):
.jpg)
Such a chemical compound is known equally a crosslinker for it produces, on reaction with the resin, a three-dimensional structure like to the polyurethane formed from a polyol and an isocyanate.
When these 2 components are mixed together, a chemic reaction takes identify between the hydroxyl groups on the polymer (acrylic resin) and the isocyanate groups on the cantankerous linker:
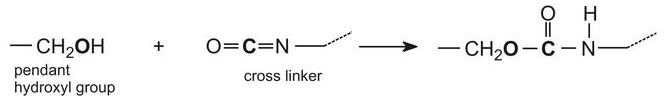
This reaction proceeds relatively slowly at room temperature, allowing enough fourth dimension for the paint to be practical, after which the solvent thinner evaporates and the painted item is placed in an oven to advance the chemical reaction. This greatly increases the molecular mass of the polymer causing it to go a three-dimensional molecule and grade a hard film, resistant to chemicals.
.jpg)
Alkyd polymers (resins)
Decorative gloss paints typically incorporate alkyd polymers (resins). A typical resin is that produced from a polyol such as propane-1,ii,3-triol (glycerol) with a dibasic acid such every bit benzene-1,2-dicarboxylic (phthalic) anhydride and a drying oil (linseed or soybean oil). On being heated together, ester linkages are formed, and water is a past-production. The proper name alkyd is derived from booze and anhydride.
The first footstep in making the alkyd polymer is the reaction betwixt the triol and the drying oil to produce a monoglyceride. For example:
.jpg)
The monoglyceride then reacts with the anhydride to grade the alkyd polymer (resin):
.jpg)
The alkyd resins, which generally have relative molecular masses in the range of 10 000 - fifty 000, are usually carried in organic solvents (solvent-borne paints). Turpentine extracted from trees was used in the by every bit the solvent, only this has been replaced by solvents from petrochemical feedstock, such as 'white spirit' which is a mixture of aliphatic and alicyclic hydrocarbons.
Once the alkyd resin is applied, the pendant oil drying groups react with oxygen in the air to course a cantankerous-linked, difficult thermoset coating, with a high molecular mass.
Epoxy polymers (resins)
Epoxy resins are frequently used equally the binder in industrial coatings (primers). They give the pigment excellent adhesion together with high resistance to chemicals (corrosion), and physical resistance necessary, for example, on ships and chemical storage tanks.
The epoxy resins are fabricated from 1-chloro-ii,3-epoxypropane (produced from 3-chloropropene) and substituted phenols, such equally bisphenol A:
.jpg)
The value of north tin be controlled to give a range of resins varying from viscous liquids to solids with high melting points. Epoxy resins tin be carried in solvents such as aromatic hydrocarbons, alcohols, ketones and esters (solvent-borne paints) or every bit dispersions in water (water-borne paints) as true emulsions. They are not normally used in topcoats for outdoors because they are susceptible to UV deposition, but they make fantabulous interior coatings and exterior primers.
Epoxy resins are also used equally adhesives (e.one thousand. Araldite) and electric insulators.
Pigments used in paints
Pigments requite colour and opacity to paints. Amongst the organic pigments, particularly important are azo-, phthalocyanine and anthraquinone derivatives.
The most mutual inorganic pigment is white titanium dioxide (titanium(Four) oxide) which provides over 70% of total pigments used (Unit of measurement 51). It has a high refractive index and gives a 'gloss' to the pigment. Another widely used inorganic paint is finely divided calcium carbonate. This has a depression refractive index and is used, together with titanium dioxide, to produce 'matt' paints. Other pigments include fe oxides (black, yellow and ruddy), zinc oxide and carbon black.
Powdered metals such equally zinc and some metallic compounds, for example zinc phosphate, have corrosion inhibiting properties.
Pigment drying
As the paint dries, a film is formed which adheres to the surface of the cloth to which it is being applied.
Emulsion paints dry by a physical process involving the evaporation of h2o followed by coalescence of the polymer droplets and their subsequent integration into a hard polymer matrix that acts equally a binder for the paint.
On applying gloss paints, the alkyd polymer cross-links by an oxidation reaction with oxygen in the air in one case the solvent has largely evaporated. This reaction is accelerated using salts of transition metals (for case, cobalt and manganese naphthenates). The transition element ion (with variable oxidation state) catalyses cross linking of the polymer chains, producing a hard surface pic to the paint.
Properties of an ideal pigment
These vary greatly according to the particular stop use. The requirements for an automotive topcoat, for example, will be very different to those for a decorative ceiling pigment.
Some of the typical attributes required can include:
- ease of application
- adept period out of awarding marks (e.g. brush-mark)
- forming a continuous protective film
- loftier opacity
- quick drying
- corrosion resistance
- h2o resistance
- heat resistance
- colour stability (i.e. against visible and ultraviolet radiation)
- chafe and scratch resistance
- durability
- flexibility
- easily cleaned
| Effigy iv These are weathering racks. Paints have been applied to panels and are exposed, at angles of 45° to the horizontal and south facing, to assess durability. Amongst the properties that are monitored are: color alter (fading), gloss change, dirt option-upwardly, nifty, flaking and contamination by fungi and algae. By kind permission of Q-Lab Europe Limited. | |
Application methods
Numerous methods are used including: brush, roller, dipping, flowcoating, spraying, hot spraying, electrostatic spraying, airless spraying, electrodeposition, pulverization blanket, vacuum impregnation and immersion.
Environmental problems
Lead compounds are no longer used in decorative paints and automotive paints. The quantity of lead compounds still being used in specialised industrial paints has been greatly reduced and somewhen alternatives volition be found. This as well applies to chromates which, although they perform well and in the past have been extensively used on motor vehicles, are very toxic.
Considering volatile hydrocarbons tin atomic number 82 to pollution in the troposphere, coatings with lower organic solvent content are required. The routes to achieve this include:
- water-based polymers (emulsion paints)
- higher solids content polymers (using less solvent)
- powder coatings
H2o-based gloss paints are at present available but the initial gloss of the finish is usually not as high every bit organic solvent based paints. The client's choice is between a high performance product and a more than environmentally friendly one. Intense inquiry attempt continues to ameliorate these paints.
High solids paints (which are solvent-based) are at present available but not without compromises in cost and performance. The relative molecular masses of the polymer resins are reduced to a maximum of ca 1000 compared to 5000 in conventional paints. This allows the proportion of the polymer to be increased from 20-30% to twoscore%, hence the term loftier solids. The main problem is the demand to maintain a low viscosity. As the corporeality of solids increases so does the viscosity, reaching a point at which the pigment cannot be applied properly. The lower proportion of solvent tends to wearisome downward the drying and motion-picture show hardening process, so changes are made to the structure of the polymer - increased branching tends to reduce viscosity for the same molecular mass. The application of the paint is more difficult. If applied by aerosol, the paint has to be under pressure. Sometimes the pigment is applied hot. It is difficult to go equally adept a terminate in advent using a high solids paint.
| Figure 5 Spraying a ship in dry out dock. The lower part is ofttimes coated with paints containing a silicone (Unit 68) or a fluoropolymer (Unit 66) which prevent barnacles attaching to the ship and and then reduces friction leading to reduced energy costs. A fouled ship can suffer a 40% increase in fuel consumption. |  |
Powder coatings are used in particular for goods such every bit bicycles and white goods (refrigerators, washing machines). The powder is fabricated upwards of a resin (frequently an epoxy resin), pigments, a catalyst to promote cantankerous-linking when the powder is heated, and additives. The pulverization is sprayed on to the article using an electrostatic spray gun and is then heat cured to produce a hard blanket. Recently acrylic powder coatings take been introduced equally clear-coats on car bodies. Although an ideal solution for many applications, curing is achieved at loftier temperature in an oven and is therefore not universally applicable (east.1000. painting of wood and plastics).
Date last amended: 18th March 2013
Source: https://www.essentialchemicalindustry.org/materials-and-applications/paints.html
Posted by: lavalleydespassoling.blogspot.com


0 Response to "How To Make Paint With Chemicals"
Post a Comment